
One contains the axis, and one contains the perpendicular. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau. Combining the out-of-phase orbitals results in an antibonding molecular orbital with two nodes. Orbital diagrams are a visual way to show where the electrons are located within an atom. Side-by-side overlap of each two p orbitals results in the formation of two π molecular orbitals. For the out-of-phase combination, there are two nodal planes created, one along the internuclear axis and a perpendicular one between the nuclei.įigure 7.7.6. Electrons in this orbital interact with both nuclei and help hold the two atoms together, making it a bonding orbital.
#Orbital diagrams download
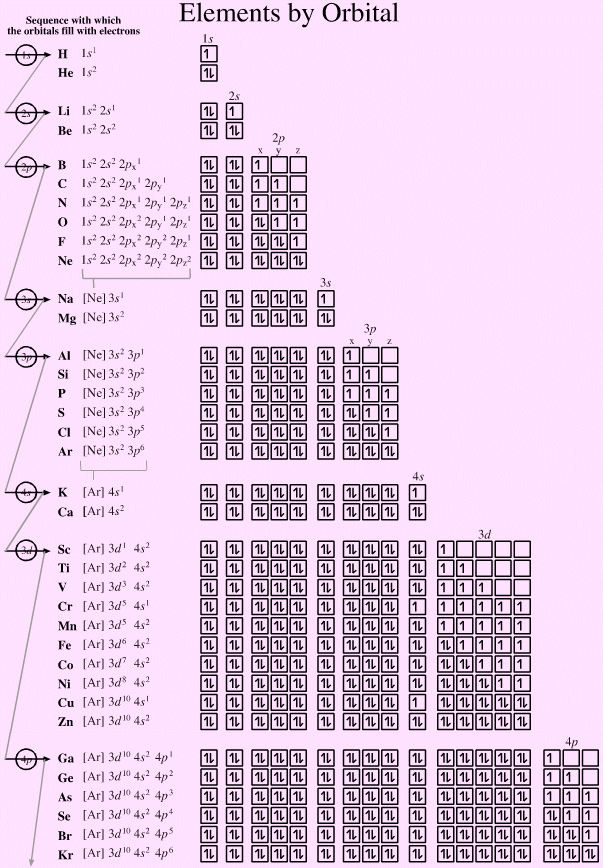
In molecular orbital theory, we describe the \pi orbital by this same shape, and a \pi bond exists when this orbital contains electrons. Download scientific diagram FIG URE 5 Molecular orbital diagrams of MB n from publication: Lanthanide metals in the boron cages: Computational prediction. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. A box, line, or circle, is drawn to represent each orbital in the electron configuration. An orbital diagram provides a visual representation of the way in which an atoms electrons are distributed into various orbitals.


In valence bond theory, we describe π bonds as containing a nodal plane containing the internuclear axis and perpendicular to the lobes of the p–\pi orbitals, with electron density on either side of the node. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. The side-by-side overlap of two p orbitals gives rise to a pi (\pi) bonding molecular orbital and a \pi* antibonding molecular orbital, as shown in Figure 7.7.6. Combining wave functions of two p atomic orbitals along the internuclear axis creates two molecular orbitals, σp and σ∗p. Just as with s-orbital overlap, the asterisk indicates the orbital with a node between the nuclei, which is a higher-energy, antibonding orbital.įigure 7.7.5. There is an \ce^* (antibonding) (read as “sigma-p-x” and “sigma-p-x star,” respectively). This electronic structure adheres to all the rules governing Lewis theory.


 0 kommentar(er)
0 kommentar(er)
